
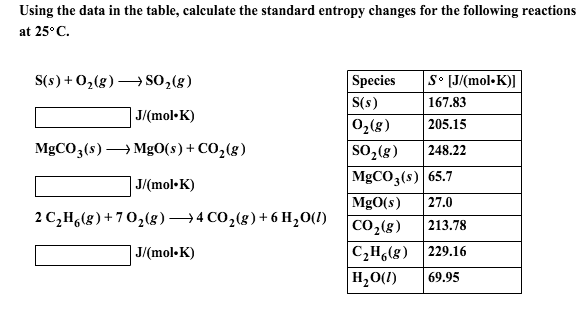
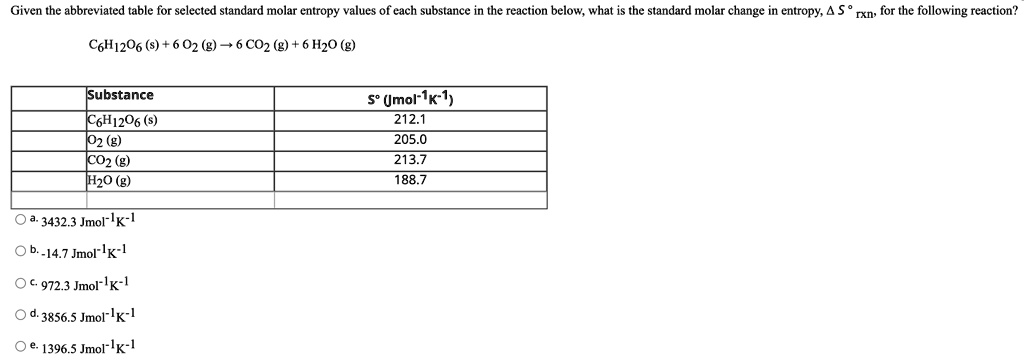
Gases tend to have much larger standard molar enthalpies than liquids, and liquids tend to have larger values than solids, when comparing the same or similar substances.There are more possible arrangements of atoms in space for larger, more complex molecules, increasing the number of possible microstates. Larger, more complex molecules have higher standard molar enthalpy values than smaller or simpler molecules.Several trends emerge from standard molar entropy data: Table 18.1c Standard Molar Entropies of Selected Solids at 298 K Solid Table 18.1b Standard Molar Entropies of Selected Liquids at 298 K Liquid Table 18.1a Standard Molar Entropies of Selected Gases at 298 K Gas These values have been tabulated, and selected substances are listed in Table 18.1a to c “Standard Molar Entropies of Selected Substances at 298 K”. The standard molar entropy, S°, is the entropy of 1 mole of a substance in its standard state, at 1 atm of pressure. Assume the change is reversible and the temperature remains constant. \(\Delta S_\).Determine the change in entropy (in J/K) of water when 425 kJ of heat is applied to it at 50☌. If the happening process is at a constant temperature then entropy will be Furthermore, it includes the entropy of the system and the entropy of the surroundings.īesides, there are many equations to calculate entropy:ġ. Also, scientists have concluded that in a spontaneous process the entropy of process must increase.
#Standard entropy table free#
Moreover, the entropy of solid (particle are closely packed) is more in comparison to the gas (particles are free to move). Entropy FormulaĮntropy is a thermodynamic function that we use to measure uncertainty or disorder of a system.

In addition, some microscope process is reversible. Besides, some other example of changeable phase is the melting of metals. On the other hand, blowing a building, frying an egg is an unalterable change.

#Standard entropy table movie#
Moreover, when the process is unalterable then the entropy will increase.įor example, watching a movie is a changeable process because you can watch the movie from backward. Also, even when the cyclic process is changeable then the entropy will not change. The second law of thermodynamics says that every process involves a cycle and the entropy of the system will either stay the same or increase. Get the huge list of Physics Formulas here The Second Law of Thermodynamics Furthermore, the more you increase the ball the more ways it can be arranged. So, now you can arrange the balls in two ways.
After some time you put another ball on the table. Moreover, the question here is in how many ways you can arrange this ball? The answer is one. In another example, you grab a ball and put it on a table. So, what will happen next? We all know that the smell will spread in the entire room and the perfume molecule will eventually fill the room. Suppose you sprayed perfume in one corner of the room. Furthermore, we can understand it more easily with the help of an example. Moreover, the higher the entropy the more disordered the system will become.
#Standard entropy table how to#
Furthermore, you will inspect the formula for entropy and find out how to use it in a variety of cases.Įntropy refers to the number of ways in which a system can be arranged. Moreover, you will explore the second law of the thermodynamics where entropy is introduced. Also, in this topic, we will learn about entropy, entropy formula, its derivation and solved example. Entropy is not a very familiar topic to most of the people.


 0 kommentar(er)
0 kommentar(er)
